Unit Thermochemistry Hess's Law - Wksh #6
Introduction to heat flow temperature change and specific heat. So you can calculate the enthalpy as the sum of several small steps.
Solved Homework 2 Name Date Class Period M Unit Chegg Com
Exercise 1 Internal Energy.
. C2H4 g 3 O2 g 2 CO2 g 2 H2O l H 1411. Thermochemistry Hesss Law - Wksh 6 Directions. The change to the first equation will allow us to 1 cancel out the water 2 cancel out the hydrogen and 3 cancel out one of the oxygens leaving the five we need for the answer.
The combined data for all of the lab sections is found below. Hess Law Practice Questions SURPASS TUTORS. Different substances have different specific heat values a.
C2H4 g H2 g C2H6 g from the following data. Consult your laboratory instructor if you believe yours are in error. _ Date_ Class period_ Unit.
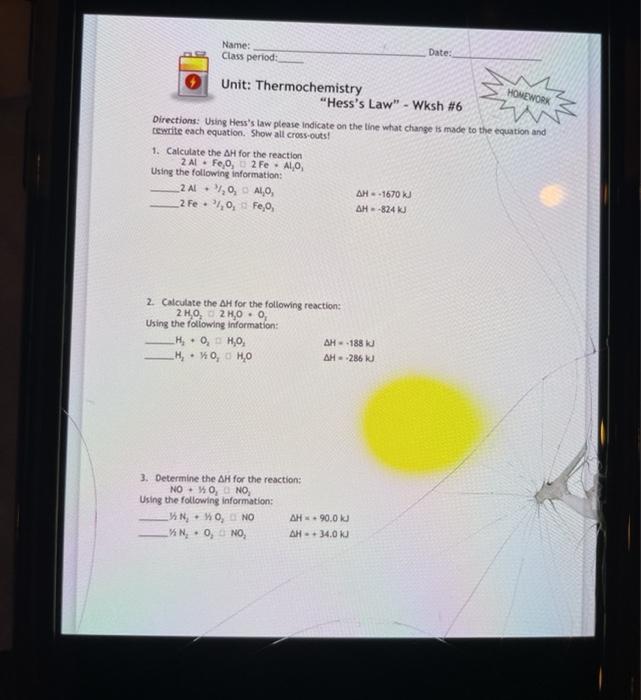
Hess helped formulate the early principles of thermochemistry. Hesss Law Worksheet answers 1. Using Hesss law please indicate on the line what change is made to the equation and rewrite each equation.
View Wksh6-HessLawpdf from SCI 101 at Campo Verde High School. Calorimetry Enthalpy in Physical and Chemical Change Hess Law Enthalpies of Formation Bond Energies. Calculate the AH for the reaction 2 AL Feo 2 Fe Alo Using the following information.
Hesss law states that the total enthalpy change does not rely on the path taken from beginning to end. Using Hesss law please indicate on. And Hesss Law of Heat Summation in order to predict the enthalpy change of the reaction.
Pressure is defined as force per unit of area so when the volume is changed work was either done on the gas or by the gas. Up to 24 cash back Lesson 3 and 4 - Thermochemical Equations and Calorimetry. KJ H2 g ½ O2 g H2O l H 2858 kJ.
In order to perform the calculations at the end of this lab there are a. C 2 H 2 g O 2 g 2 H 2 O l. Start studying Chapter 6.
Science Chemistry General Science. Sample Calorimetry Calculation for Lab 4. Chemistry questions and answers.
Calculate H for the reaction. His most famous paper which was published in 1840 included his law on thermochemistry. There are a few rules that you must follow when manipulating an equation.
Hesss law is due to enthalpy being a state function which allows us to calculate the overall change in enthalpy by simply summing up the changes for each step of the way until product is. This will change the sign of ΔH. CO2g COg 05 O2g H 483 Reaction 3 reversed and divided by 2.
When 1000 mL of 100 M HCl is mixed with 1000 mL of 100 M NaOH both initially at 211C are mixed in a two-cup calorimeter the temperature of the mixture rises to 279C. Cs O2g CO2g H 605 kJ Reaction 2 reversed and divided by 2. Solving specific heat calculations C p q m T Where Cp specific heat m mass in grams q the energy lost or gained T change in temperature To find the heat lost or gained use.
40k Unknown user Oct 22 2013 958 AM. Using Hesss law please indicate on the line what change is made to the equation and rewrite each equation. Thermochemistry Hesss Law - Wksh 6 Directions.
Thermochemistry Energy Enthalpy Calorimetry Hesss Law. Find the standard molar enthalpy for the reaction C s ½ O 2g CO g Given that C s O 2g CO. Hess law also known as Hess law of constant heat summation states at constant temperature heat energy changes enthalpy- ΔHrec accompanying a chemical reaction will remain constant irrespective of the way the reactants react to form product.
KJ C2H6 g 3½ O2 g 2 CO2 g 3 H2O l H 1560. The combustion of ethene C 2 H 4 is an exothermic reaction. Use a separate piece of paper to show your work.
Acids and Bases - pH pOH ph Scale Hydrogen ion concentration Hydroxide ion concentration This lesson plan will give your students a basic overview of calculations with acids and bases as well as the pH and pOH scales. Calculate the AH for the reaction 2 AL Feo 2Fe A10 Using the following information. AP Chemistry Thermochemistry Worksheet 3-.
Please solve the following problems using dimensional analysis. Thermochemistry and Hesss Law Lab Results. Q C p m T 5.
ID Lab Group Initials DHa DHb Timestamp. Up to 24 cash back Intro to Thermochemistry 3 Energy Unit Conversions Specific Heat Calculating Heat Specific Heat of Metal Calorimetry Constant Pressure Calorimetry Food Calorimetry Explanation Food Calorimetry Calculations Thermochemical Equations Parts of Heating and Cooling Curves Heating Curve Calcuations Hesss Law. Calorimetry Enthalpy and Hess Law 1.
The unit of specific heat is Jg oC 4. D 2 Mg s O 2 g 2 MgO s. You can always check your answer using molar enthalpies of formation Ho f 1.
You can reverse the equation. The H for the reaction as written is. Thermochemistry Thermochemical Equations Wksh 3 Directions.
ΔH -5716 kJ. Demonstrates Hesss law using the production of acetylene gasChem 1090 Hess 4. 2 AL 70 A10 2 Fe 20 Fe0 AH -1670 kJ AH -824 kJ 2.
Hesss Law Practice Problems Answers Determine Ho for each of the following problems. Detailed Class Notes Calorimetry Theory. Determine the heat produced by a chemical or physical process from experimental data.
AP Chemistry Thermochemistry Worksheet 2--Calorimetry and Heat Capacitydoc. Thermochemistry Hesss Law Wksh 6 HOMEWORK. Unit 3 - Thermochemistry - Topics.
Determine the H of neutralization for the reaction HClaq NaOHaq NaClaq H2Ol. Learn vocabulary terms and more with flashcards games and other study tools. 2 AL 701 Algo AH -1670 kJ 2 Fe 0 FeO AH.
2N2 g6O2 g2H2 g4HNO3lH -6964kJ. For a system undergoing an endothermic process in which 156 kJ of heat flows and where 14 kJ of work is done on the system.

Solved Name Date Class Period Unit Thermochemistry Chegg Com

Solved Name Date Class Period Unit Thermochemistry Chegg Com
No comments for "Unit Thermochemistry Hess's Law - Wksh #6"
Post a Comment